Low Risk of Pathogens in Intentionally-Produced Raw Milk
Despite raw milk’s association with decreased rates of asthma, allergies, eczema, ear infections, fever, and respiratory infections, government agencies in countries such as the USA, Canada, and Australia are still biased against raw milk. These Government agencies warn against raw milk consumption and, in some places, they even impose an outright ban on raw milk with potential heavy penalties and imprisonment for raw milk farmers.
The rationale that these Government agencies cite against raw milk is their belief that raw milk consumption leads to high rates of foodborne outbreaks, illnesses and deaths. However, this belief is outdated and conflicts with the most up-to-date peer-reviewed research which has found that carefully produced raw milk is a low-risk food which is fundamentally different from pre-pasteurized milk.
The table below contrasts pathogen test data from pre-pasteurized milk vs. carefully-produced raw milk intended for direct human consumption. As illustrated in the table, pathogen testing of pre-pasteurized milk samples has detected pathogens in up to 33% of samples. In contrast, there were zero pathogens detected in thousands of milk samples from raw milk intended for direct human consumption. It is clear from this test data from bulk tanks or milk silos that the risk profile of pre-pasteurized milk is categorically different from raw milk intended for direct human consumption.
Data courtesy British Columbia Herdshare Association
Pathogen Loads and Illness
Carefully-produced raw milk has a low-risk of containing pathogens, but there is no such thing as a perfectly safe food. A CDC analysis of foodborne illnesses from 2009-2015 showed that the top food categories commonly linked to illnesses were chicken, pork, and seeded vegetables. Pasteurized milk is not perfectly safe, either, and is implicated in foodborne illnesses and outbreaks.
In the very rare case that a pathogen could be present in carefully-produced raw milk, in order for a pathogen to cause illness four variables must align:
A pathogen must be present
The pathogen must be virulent and capable of producing harmful effects
The pathogen load must be high enough to produce illness
The person must be susceptible to the pathogen
If it is present in a small enough quantity, even the most virulent pathogen will not produce illness. The presence of a single virulent bacterium is not sufficient to cause illness, and different pathogens have varying thresholds at which they must be present to induce human illness.
For instance, even though Listeria monocytogenes is a known foodborne pathogen, the European Union allows Listeria up to 100 bacteria/gram in foods that do not permit growth because it is known that Listeria in lesser amounts is not sufficient to cause illness.
Some of the data cited by Government agencies against raw milk includes pathogen growth studies where it was found that pathogens multiply greatly over time. However, these studies are not actually applicable to carefully-produced raw milk because they were performed in nutrient-rich broth instead of milk, they used tremendously high amounts of pathogens (such as 10 log 7, which corresponds to ten million pathogenic colony-forming units (CFU) of bacteria per mL), or they did not account for cold temperature storage.
Need for a NEW Pilot Study on Pathogen Growth for Raw Milk
In order to generate a stronger scientific basis for assessments of risks of pathogen growth in raw milk, the Raw Milk Institute (RAWMI) recently commissioned a pilot study on pathogen growth performed by an independent 3rd party lab certified to perform pathogen testing, Food Safety Net Services (FSNS). RAWMI Advisory Board member Peg Coleman provided technical input on the study design based on predictive microbiology (Coleman et al., 2003a) and risk assessment (Coleman et al., 2003b) studies that she had conducted at the University of Maryland Eastern Shore and published through the USDA Agricultural Research Service. The new pilot study was partially paid for through donations.
In this new pilot study, samples of well-produced raw milk were purposely inoculated with the four main pathogens of concern for raw milk: E coli 0157:H7, Salmonella spp., Campylobacter spp., and Listeria monocytogenes. Raw milk was inoculated at two levels (high and moderate counts per mL). The objective of this new pilot study was to document growth characteristics of these pathogens in carefully produced raw milk over a period of 14 days when stored at the refrigeration temperature recommended by FDA and USDA: 40°F (4.4 °C). The number of pathogenic bacteria present in the raw milk were counted on days 0, 3, 6, 9, 12, and 14.
Highlights of NEW Pilot Study Design
The temperature for this study was chosen because 40°F (4.4°C) is the recommended maximum temperature for a home refrigerator.
Inoculum Level I: target <10 CFU/mL. Although the study design called for inoculation with <10 CFU/mL, the actual amounts used in the study were measured in the range of 22-162 CFU/mL, thus a moderate level inoculum.
Inoculum Level II: target 1,000 CFU/mL. Although the study design called for inoculation with 1,000 CFU/mL, the actual amounts used in the study were measured in the range of 600-8,300 CFU/mL.
Results of the NEW Pilot Study
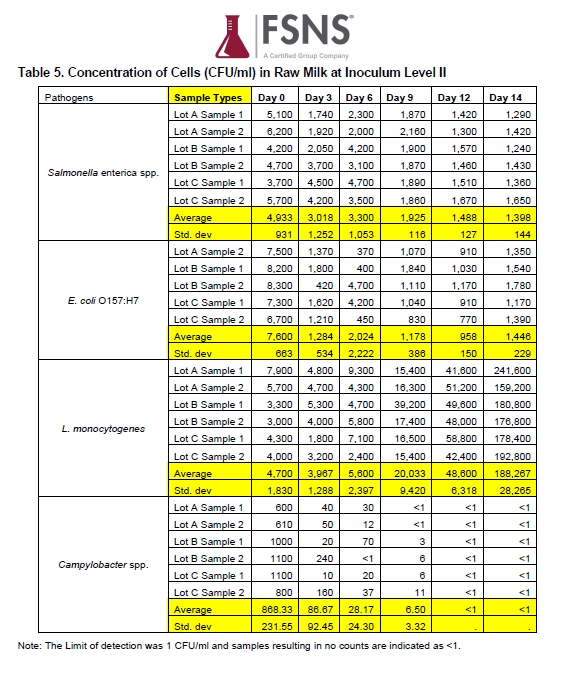
The tables below show the results of the study at Inoculum Levels I and II.
Table of Results from Inoculum Level I, from FSNS Report, Determination of Growth Rate of Salmonella enterica spp., E. coli O157:H7, Campylobacter spp., and Listeria monocytogenes in Raw Milk
Table of Results from Inoculum Level II, from FSNS Report, Determination of Growth Rate of Salmonella enterica spp., E. coli O157:H7, Campylobacter spp., and Listeria monocytogenes in Raw Milk
The most relevant finding of the study is that at moderate Inoculum Level I, no pathogen growth is observed through at least 6 days of refrigerated storage. The very high Inoculum Level II results are less important to risk assessors since these levels of pathogens are not observed in naturally contaminated raw milk.
Over the study period of 14 days, the counts per mL of E coli 0157:H7, Salmonella spp., and Campylobacter spp. decreased over time. These results indicate that, when stored at the recommended refrigerator temperature, moderate to high counts of E coli 0157:H7, Salmonella spp., and Campylobacter spp. did not multiply over time in raw milk. Listeria monocytogenes exhibited some growth in this study after 9 days of refrigeration at both moderate and high level inoculum levels.
Click the button below to download the full report from FSNS, Determination of Growth Rate of Salmonella enterica spp., E. coli O157:H7, Campylobacter spp., and Listeria monocytogenes in Raw Milk.
Further Research
This study was designed as a small pilot study, and further research is needed to draw more-robust conclusions. Analysis of the NEW pilot study data are in preparation for submittal to a peer reviewed journal. Peg Coleman will also be providing a more detailed analysis of the study.
The new pilot study and the publication are intended to support a grant proposal to fund a full study that includes multiple producers of raw, lightly pasteurized, and typical pasteurized milks, with daily sampling after low and high inoculum levels. Nonetheless, the results of this NEW pilot study serve to provide an initial basis for challenging incorrect assumptions of the past that overestimated the growth of pathogens in clean, cold raw milk produced for direct human consumption by careful, trained producers.