Now, with access to PMP, the risk assessor can select the inputs from those tested in multiple factor broth culture experiments from the sliders illustrated in the screen shot from PMP on the left. I illustrated a growth scenario with an appropriate refrigeration temperature (5°C or 41°F, from a range of 5-42°C or 41-107.6°F) and a pH (6.5, from a range of 4.5-8.5) relevant to raw milk.
The first problem for dairy farmers and raw milk consumers is that models based on optimal growth of pathogens in pure cultures described by rich broth culture models overestimate actual pathogen growth in raw milk. As early as 1997, university researchers published experimental results reporting that the rate of growth of the pathogen E. coli O157:H7 was significantly slower in raw milk than pasteurized (Wang et al., 1997). The authors noted that the difference in growth rates was likely due to the natural microbes in raw milk that outcompete pathogens and limit their growth in raw, not pasteurized, milk.
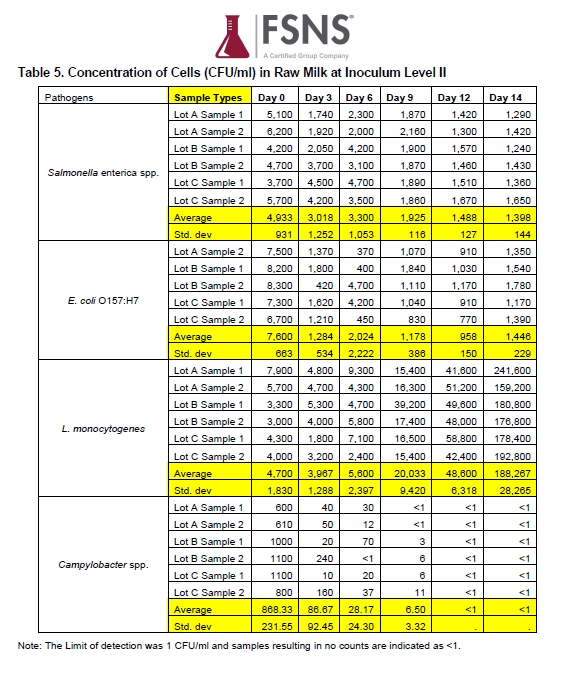
Another problem for farmers and consumers is that the broth culture study designs are typically biased by inclusion of only high initial pathogen levels (> 3 log10 colony forming units (CFU) per mL or >1,000 CFU/mL, from a range of 3 to 5.9 log10 CFU/mL). Even in rich culture broth, growth rates are lower at low inoculum levels (~1 CFU/mL; Coleman et al., 2003). Biased growth models (based on rich nutrient broth, high initial inoculum, and/or absence of natural milk microbiota) result in biased MRAs that overestimate raw milk risks.
You may not be surprised to learn that some microbial risk assessment teams, including the Food Standards Australia New Zealand team (FSANZ, 2009), selected rich culture broth studies (Salter et al., 1998; Ross et al., 2003) that measured growth of harmless or commensal E. coli strains that are part of our healthy gut microbiota, not even pathogenic strains like O157:H7 that can cause illness and grow at slower rates. FSANZ excluded an available study on growth of the pathogen E. coli O157:H7 itself in raw and pasteurized milk reported by Wang and esteemed food scientist Mike Doyle at the University of Georgia (Wang et al., 1997).
Why do you think the FSANZ team decided not to cite Mike Doyle’s study, a study they should have known about? Likely because it measured lower pathogen growth rates in raw milk than in pasteurized milk (and broth). Thus, it seems that FSANZ likely excluded the study because the results did not support their notion that raw milk is inherently dangerous, and more dangerous than pasteurized milk. A short plain language summary prepared by the Australian Raw Milk Movement (ARMM) and the full 73-page technical report that I prepared for them (Coleman, 2021) are both available on the ARMM website. See the technical report for the more detailed section on pathogen growth and microbial ecology (pp. 30-40 of the 73-page report).
Why is Inoculum Level Important to Predict Growth in Raw Milk?
Well-produced raw milk has relatively low levels of coliform and aerobic bacteria. Farmers who follow RAWMI’s Common Standards for raw milk aim for coliform counts of <10 CFU/mL and Standard Plate Counts of <5,000 CFU/mL. However, don’t let these low coliform counts or low Standard Plate Counts in raw milk fool you.
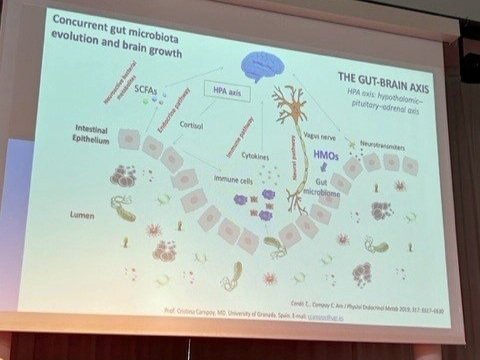
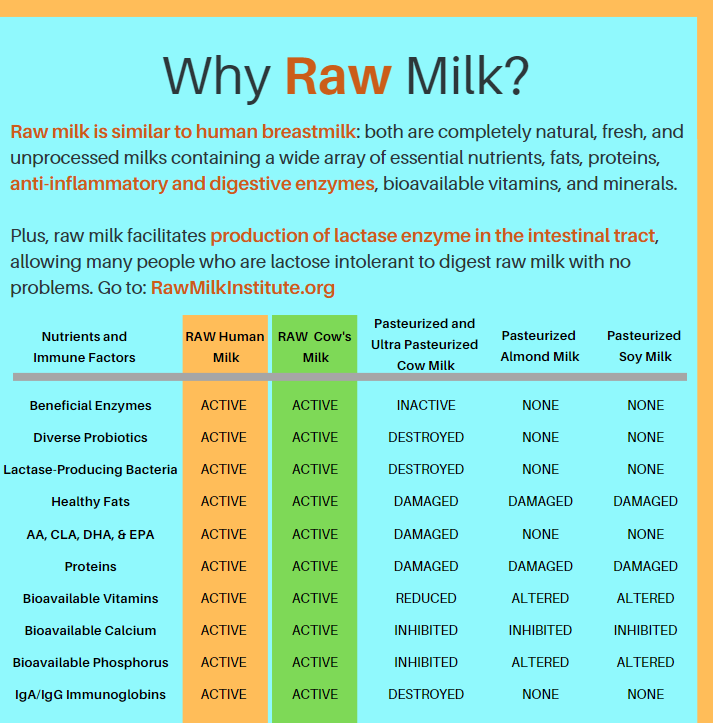
Raw mammalian milks are complex ecosystems with dense and diverse microbes that benefit health. The natural microbes in raw milks have different requirements for culturing them, so studies that rely on specific culture media for assessing what microbes are present in raw milk are biased. The development of genomic methods that estimate presence of microbial genes or gene products in raw milks without culturing are more reliable for describing the raw milk microbes or microbiota (Oikonomou et al, 2020). Such studies are transforming our understanding of the microbiota of many natural systems in the recent decade, including raw mammalian milks.
The dense and diverse microbiota predominant in raw milk from healthy mammals is illustrated in the figure below by Oikonomou and colleagues (2020; authors’ Figure 2, pg. 4 of 15). The bacteria listed in red text were identified in the milk microbiota from all five types of mammals, bacteria in yellow from 3 or more mammals, and bacteria in blue in less than three mammals. None of these bacteria were identified as pathogens, but rather are natural microbes that appear to benefit human and animal offspring (and adult humans) by ‘seeding and feeding’ the gut. In other words, raw milk ‘seeds’ the gut with beneficial microbes and ‘feeds’ gut and microbial cells with nutrients. The raw milk microbiota also stimulates proper maturation and function of immune, neural, and respiratory systems (Coleman et al., 2021a,b; Dietert et al., 2022).